What Element Is a Nonmetal With One Valence Electron
Now that we know the electron configuration of the valence electron in sulfur is 3p4 based on its position in the periodic table and we have a picture of how those p electrons are filling the p sublevel the set of quantum numbers for this valence electron are extremely easy to obtain. The electrons in the outermost shell are the valence electrons the electrons on an atom that can be gained or lost in a chemical reaction.
What Are Non Metals With One Valence Electrons Quora
The species Mn Co and Se are not at the edge of their blocks and therefore have.
. This interactive periodic table of element groups arranges the chemical elements according to periodicity or common properties. Element Electron configurations Mg 12e 1S 2 2S 2 2P 6 3S 2 Reactive Mg 2 10e Ne Stable Cl17e 1S 2 2S 2 2P 6 3S 2 3P 5 Reactive Cl-18e Ar Stable. CHEMICAL BONDS attractive force holding atoms together Single Bond.
Only carbon has the s 2 outer configuration which accounts for some of the differences between carbon and other elements in the family. Two of these electrons are in the s subshell while 2 are in the p subshell. Next it is a p.
In the periodic table potassium is one of the alkali metals. All the matter in the universe is composed of the atoms of more than 100 different chemical elements which are found both in pure form and combined in chemical compoundsA sample of any given pure element is composed only of. Oxygen a chemical element with the symbol P and atomic number 15 is a highly reactive nonmetal and a very good oxidizing agent which readily forms oxides which most of elements as well as compoundsAt standard temperature and pressure two oxygen atoms bind to form colorless odorless diatomic dioxygen gas which constitutes 21 of the earths surface.
A atomic number and therefore charge on the nucleus nuclear or core charge increases b number of valence electrons increases c atomic radius decreases d first ionisation energy increases f electronegativity increases excluding neon g elements on the left are metals elements on. Involves two electron pairs eg. The electrons on an atom that are not present in the previous rare gas ignoring filled d or f subshells.
A period 4 element is one of the chemical elements in the fourth row. H 2 Double Bond. Use protons neutrons and electrons to build elements.
All of the alkali metals have a single valence electron in the outer electron shell which is easily removed to create an ion with a positive charge a cation which combines with anions to form salts. When you add an electron you get a negatively charged. Each element is classified as a metal metalloid or nonmetal and its state at room.
Ionic bonds occur between metals and nonmetals when there is a large difference in electronegativity. Chemical compound any substance composed of identical molecules consisting of atoms of two or more chemical elements. Since there is no rigorous definition of a nonmetal some variation may be encountered among.
A paramagnetic element is one whose electron configuration contains any unpaired electrons so they are those elements that are not at the right-hand edge of a given block s p d f of the periodic table. Of these options Kr and Ca are at the right-hand edges of the p and s block respectively. A nonmetal is a chemical element having among other properties a relatively low density and moderate to high electronegativityBroadly they lack a preponderance of more metallic attributes such as luster deformability good thermal and electrical conductivity and low electronegativity.
Phenomenon unseen in lighter elements. O 2 Triple Bond. Pure covalent bonding only occurs when two nonmetal atoms of the same kind bind to each other.
Carbon family elements contain atoms that have 4 electrons in their outer energy level. When you add an electron you get a positive charge because adding is positive in math. Contrariwise the six elements from gallium to krypton are the heaviest where all electron shells below the valence shell are filled completely.
As the number of protons neutrons and electrons changes information such as the name and symbol of the element the Z N and A numbers the electron dot diagram and the group and period from the periodic table are shown. Science Tech Math Science Math Social Sciences Computer Science Animals Nature Humanities History Culture Visual Arts Literature English Geography Philosophy Issues Languages English as a Second Language. Naturally occurring potassium is composed of three isotopes of which 40K is.
As you move down the periodic table in the carbon family the atomic. Since filled d or f subshells are seldom disturbed in a chemical reaction we can define valence electrons as follows. He explains the formation of a cation like this.
Pletely transferred from one atom to the other atom. Involves three electron pairs eg. If complete octets cannot be formed when using only single bonds between atoms it is necessary to draw multiple bonds.
The following general trends are observed as you go across period 2 from left to right. Involves an electron pair eg. Ionic Bonding In COVALENT BONDING the valence electrons are shared as pairs between the bonded atoms.
First n 3 since it is a 3p electron. This is no longer possible in further periods due to the existence of f-subshells starting from n 4. One of your classmates is having trouble understanding ions.
8 e total valence electrons1 atom of O6 valence e3 atoms of H1 valence e1 valence e17 e When is it necessary to draw a multiple bond in an electron-dot formula. As a group explain in a grammatically correct sentence why this student is incorrect.
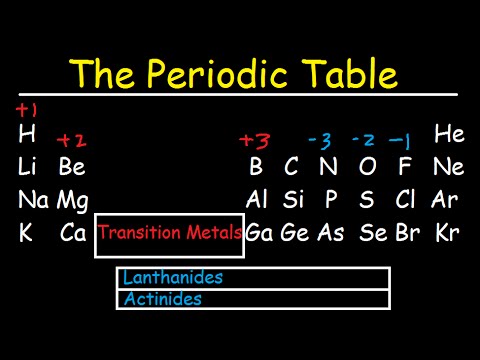
Periodic Table Of Elements Explained Metals Nonmetals Valence Electrons Charges Youtube

Comments
Post a Comment